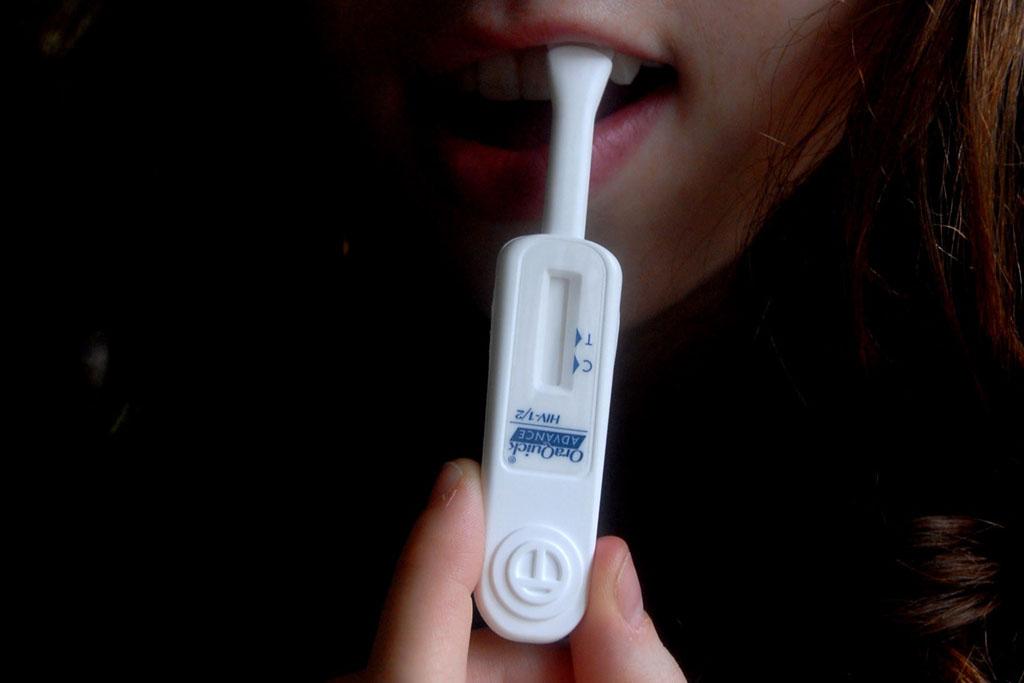
HIV at-home test approved by FDA panel
An FDA expert panel has recommended the approval of HIV home test Oraquick.
HIV home test 'Oraquick' has been recommended for approval by the FDA after an expert panel gave it the go-ahead Tuesday.
The FDA advisory panel voted 17-0 to approve the at-home test for the virus, which causes AIDS, after determining that it was safe for the public.
The FDA will make a final decision on the product later this year but the expert panel is expected to weigh substantially on the verdict, reported the Associated Press.
The opinion of the panel was summarized by member Steven W. Pipe of the University of Michigan.
"I can't get past the quarter of a million people in the U.S. who have HIV and are not tested," said Pipe, according to WebMD.
Read more on GlobalPost: Antivirus software used in HIV vaccine research (VIDEO)
"If we make any dent in that, the answer is yes, we realize the [OraQuick At-Home] benefit outweighs its risks."
The test is manufactured by Pennsylvania-based Orasure and was approved for use by doctors in 2004.
It uses a swab of the gums to test for HIV and takes about 20 minutes for results.
The company recommends confirming the results by a blood test.
The test has a 93 percent accuracy for positive results after being used in about 5,700 tests, reported AFP.
Negative results were accurate nearly 99 percent of the time, however.
Read more on GlobalPost: How to reduce HIV in Zimbabwe? Make women uglier.
Our coverage reaches millions each week, but only a small fraction of listeners contribute to sustain our program. We still need 224 more people to donate $100 or $10/monthly to unlock our $67,000 match. Will you help us get there today?