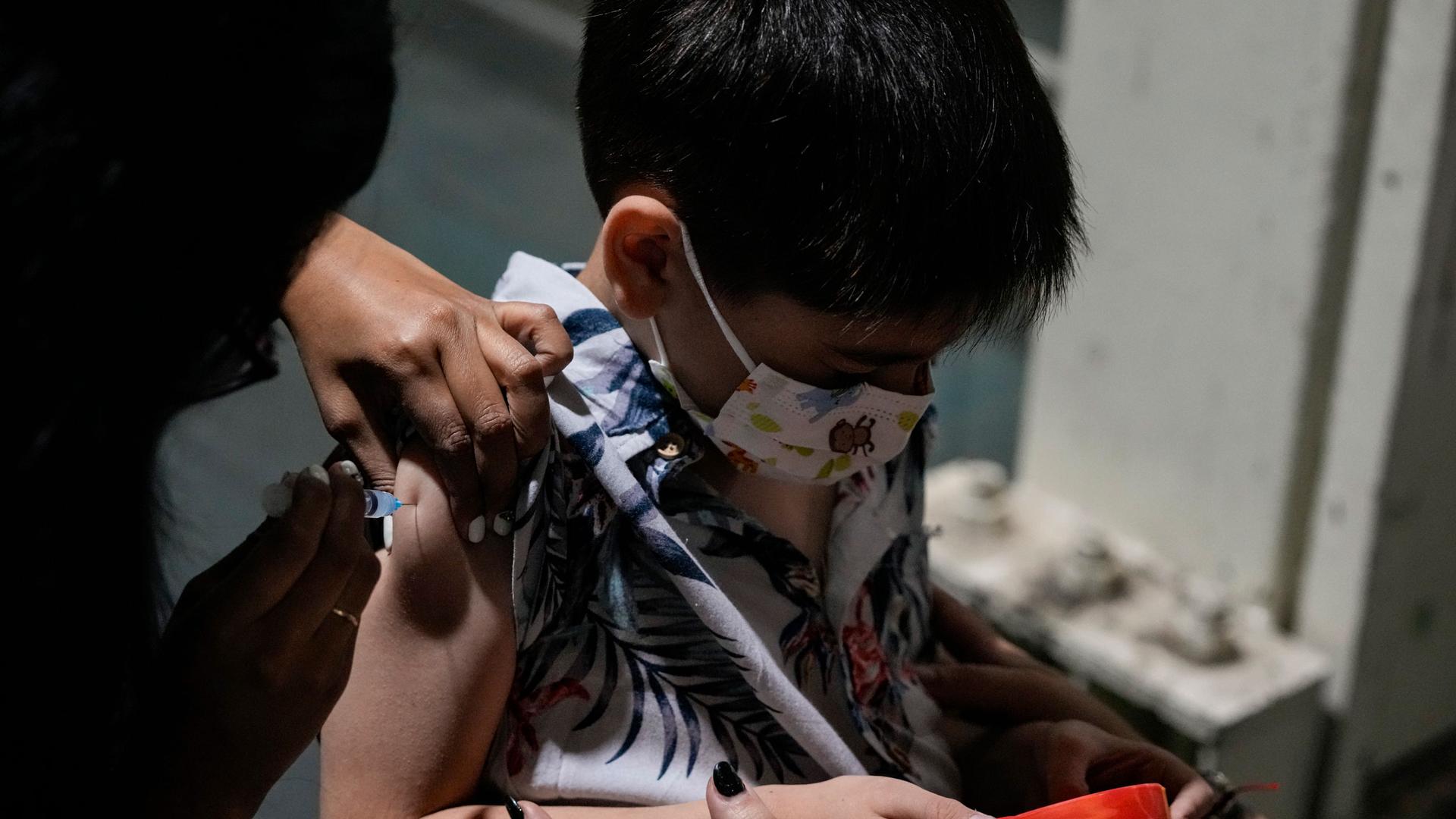
Throughout the pandemic, Shirley Monsalve González hoped for an effective vaccine for her now 4-year-old son, Pedro.
As a pediatric urologist based in the Chilean coastal city of Viña del Mar, she joined the COVID-19 response team early on at her hospital, Carlos Van Buren, in nearby Valparaíso. She worried about exposing the virus to her family, and that the virus would become as serious for children as it was for most adults.
“I felt a little bit of fear,” she told The World, in Spanish.
Around the world, including the US, COVID-19 vaccines for the very young(usually 5 and under) have been mostly unavailable throughout the pandemic, putting lots of families in a tricky spot.
But in places like Chile, very young children can already get immunized. As of early December 2021, the government opened up COVID-19 vaccinations to all children ages 3 and up.
Chile went with the CoronaVac vaccine, manufactured by the Chinese company Sinovac Biotech, for very young children.
“We are very happy because what parents want the most is that their children have a life and can be happy.”
“We are very happy because what parents want the most is that their children have a life and can be happy,” Chile’s then-President Sebastián Piñera said, sandwiched between children holding balloons at the official announcement in December last year.
In general, kids haven’t gotten as sick from COVID-19 as adults. But children may suffer from a rare inflammatory syndrome known as MIS-C, which is triggered by the virus. In the US, hospitalizations increased among the very young with the more transmissible omicron variant. And questions remain about long-COVID risks and future variants.
Related: Coronavirus Conversations: What researchers have learned about the omicron variant
Unlike the Pfizer-BioNTech and Moderna COVID-19 vaccines that use mRNA technology, the CoronaVac vaccine uses an inactivated form of the virus. Its approach is similar to vaccines used in kids for decades, like for the flu, that have been found to be safe.
For that reason, Chileans have embraced it.
“Here in Chile, the issue of vaccination is deeply rooted.”
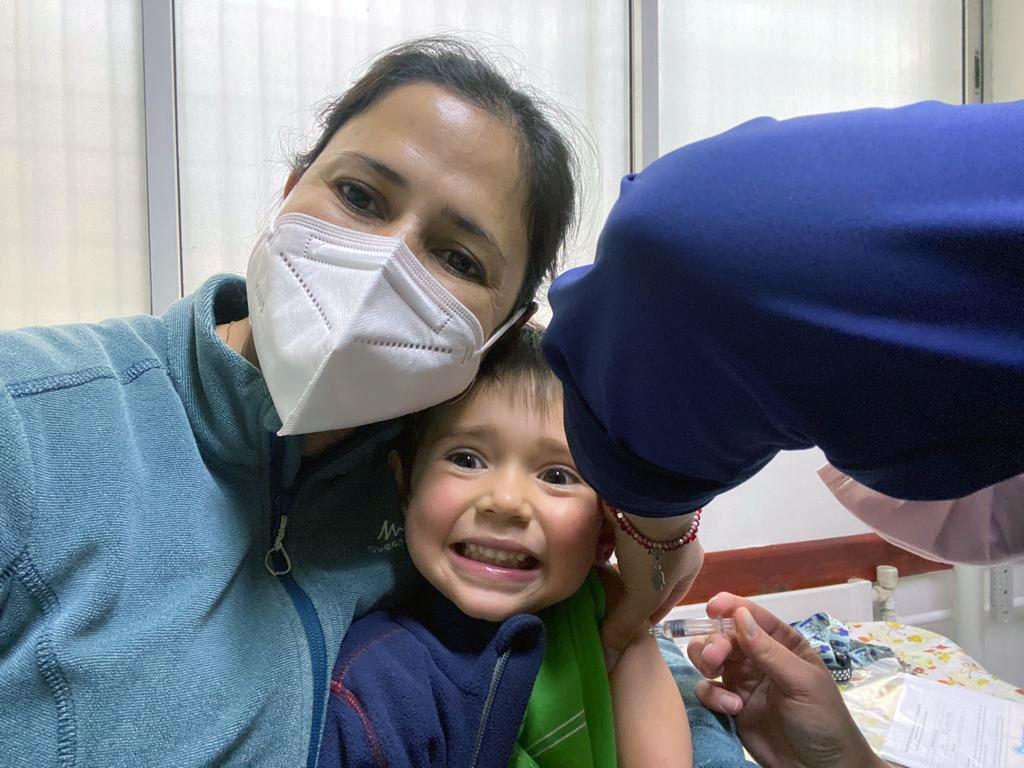
“Here in Chile, the issue of vaccination is deeply rooted,” González said, referring to the country’s strong history and culture of vaccine acceptance.
She participated in and helped oversee CoronaVac clinical trials at her local hospital in adults.
Her son is now fully vaccinated.
To date, at least 800,000 doses have been administered to children between the ages of 3 and 5 in Chile, according to the Ministry of Health.
“The vast majority of people are vaccinated, and the vast majority of children,” she said.
Chile’s overall high vaccination rate leads the world, second only the United Arab Emirates, according to an Oxford University vaccine tracker.
The Ministry of Health in Chile reviewed safety and efficacy data from earlier clinical trials of adults and children in China and Chile to make the decision to offer the vaccine to young kids, according to Alexis Kalergis, who was heading the trials in Chile.
Kalergis is also an immunology professor at Pontifical Catholic University of Chile.
“Data from [scientific-clinical trials and] studies by the Ministries of Health, some of it with omicron and the previous one with delta, suggest that the vaccine is not only safe and immunogenic, but is also is efficacious.”
“Data from [scientific-clinical trials and] studies by the Ministries of Health, some of it with omicron and the previous one with delta, suggest that the vaccine is not only safe and immunogenic, but is also is efficacious. It’s effective,” he said.
Related: Brazil heads into latest COVID surge amid public health information blackout
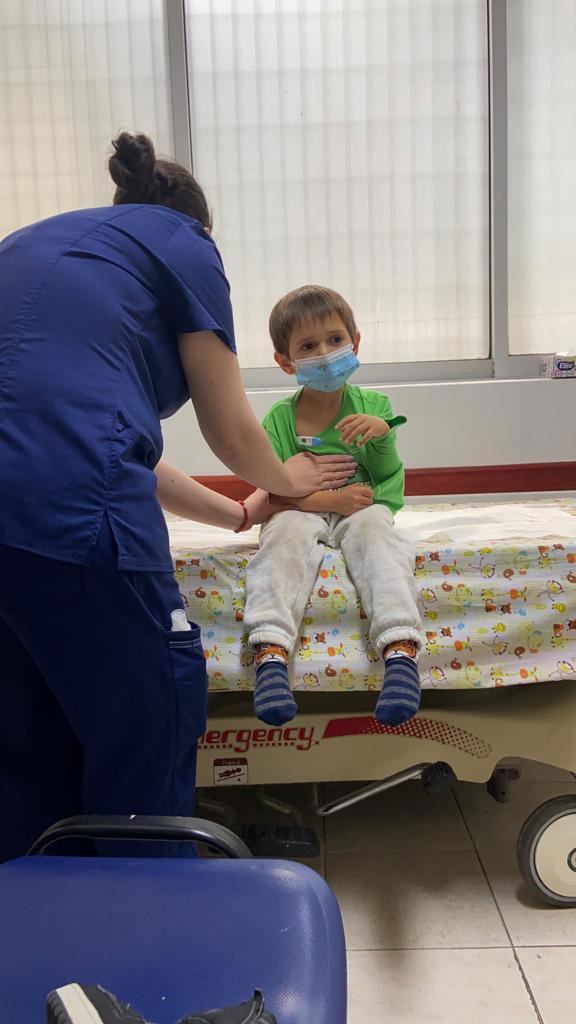
An observational analysis of hundreds of thousands of children ages 3 to 5 in Chile, between December 2021 and February 2022, estimated that a two-dose series given 28 days apart was only “modestly” protective, with about 30% effectiveness at infection prevention. However, it provided about 69% protection against severe disease and hospitalization.
It has yet to be independently reviewed.
Sinovac’s CoronaVac vaccine is widely used around the world for adults, though some preliminary studies call into question whether it produces as much protection when compared to mRNA vaccines, and how long that protection may last.
Meanwhile, the vaccine has been approved for use in children ages 3 and up in several countries, including Colombia, China and Thailand.
Argentina and Bahrain have authorized China’s Sinopharm vaccine for young kids.
In Cuba, most children ages 2 and up are vaccinated with the country’s homegrown vaccine. Venezuela is also using Cuba’s vaccine.
Kalergis said Brazil is currently reviewing Chile’s data to consider expanding CoronaVac to children under 6.
The World Health Organization has only authorized the CoronaVac vaccine for those 18 and up, stating that in general, COVID-19 vaccines are less urgent for children than they are for those in higher-risk groups like seniors, health care workers, and those with chronic health conditions.
A complicated path toward COVID-19 vaccines in young children
Finding the right approach for vaccinating young children has proven harder than for adults — and it’s also brought some unexpected results.
For example, CoronaVac protection rates appear lower in young children compared with kids ages 6 to 16.
In the US, Pfizer recently regrouped to study the effectiveness of a three-dose series, instead of two doses, in kids ages 6 months to 5.
Dr. Paul Offit, head of the Vaccine Education Center at the Children’s Hospital of Philadelphia, cautioned that the full data on COVID-19 vaccine trials in little kids around the world hasn’t been reviewed yet and that it’s difficult to draw conclusions about effectiveness.
As a member of a vaccine advisory committee to the Centers for Disease Control and Prevention, Offit said that current US trials are smaller and conducted over a shorter period of time compared to previous clinical trials, but they do follow standard protocols that ensure safety and efficacy first and foremost.
Related: At least 9 African countries set to produce COVID vaccines, Africa’s CDC chief says
The big challenge is “showing that you have the right dose, and the right dosing interval and the right number of doses. That’s really the hardest part. And it’s hard because there’s always sort of an arbitrary bracket of children’s ages,” he said.
And because COVID-19 usually doesn’t cause severe illness in kids, it’s often harder to tell if the vaccines are working. Trials conducted during the delta wave versus omicron also complicate the analysis.
Related: Israeli researchers try to find COVID ‘threshold’ with fourth vaccine dose

For Donna Farber, an immunology professor at Columbia University who has been studying the immune responses of children with COVID-19, their experiences in the pandemic raises broader questions.
“What we’re finding out is we need to understand more about children’s immune system and how it’s different than adults.”
“What we’re finding out is we need to understand more about children’s immune system and how it’s different than adults,” Farber said.
Perhaps kids may be better adapted, Farber theorized, and “have more of the sort of immune arsenal they need to respond to a new pathogen compared to an adult.”
She pointed out that, paradoxically, kids also respond much better to vaccines, in general, because their immune systems are able to build up a long-term memory to fight specific viruses.
COVID-19 underscores the need to look beyond a one-size-fits-all approach of adopting the same vaccines for children, she said.
Meanwhile, families in the US may soon be able to weigh more options for COVID-19 protection for very young children.
Moderna and Pfizer have both announced their pursuit of emergency FDA approvals this spring — if government advisers and regulators find strong data, it would make COVID-19 vaccines available for children under the age of 5.
Our coverage reaches millions each week, but only a small fraction of listeners contribute to sustain our program. We still need 224 more people to donate $100 or $10/monthly to unlock our $67,000 match. Will you help us get there today?