FDA approves new skin cancer pill
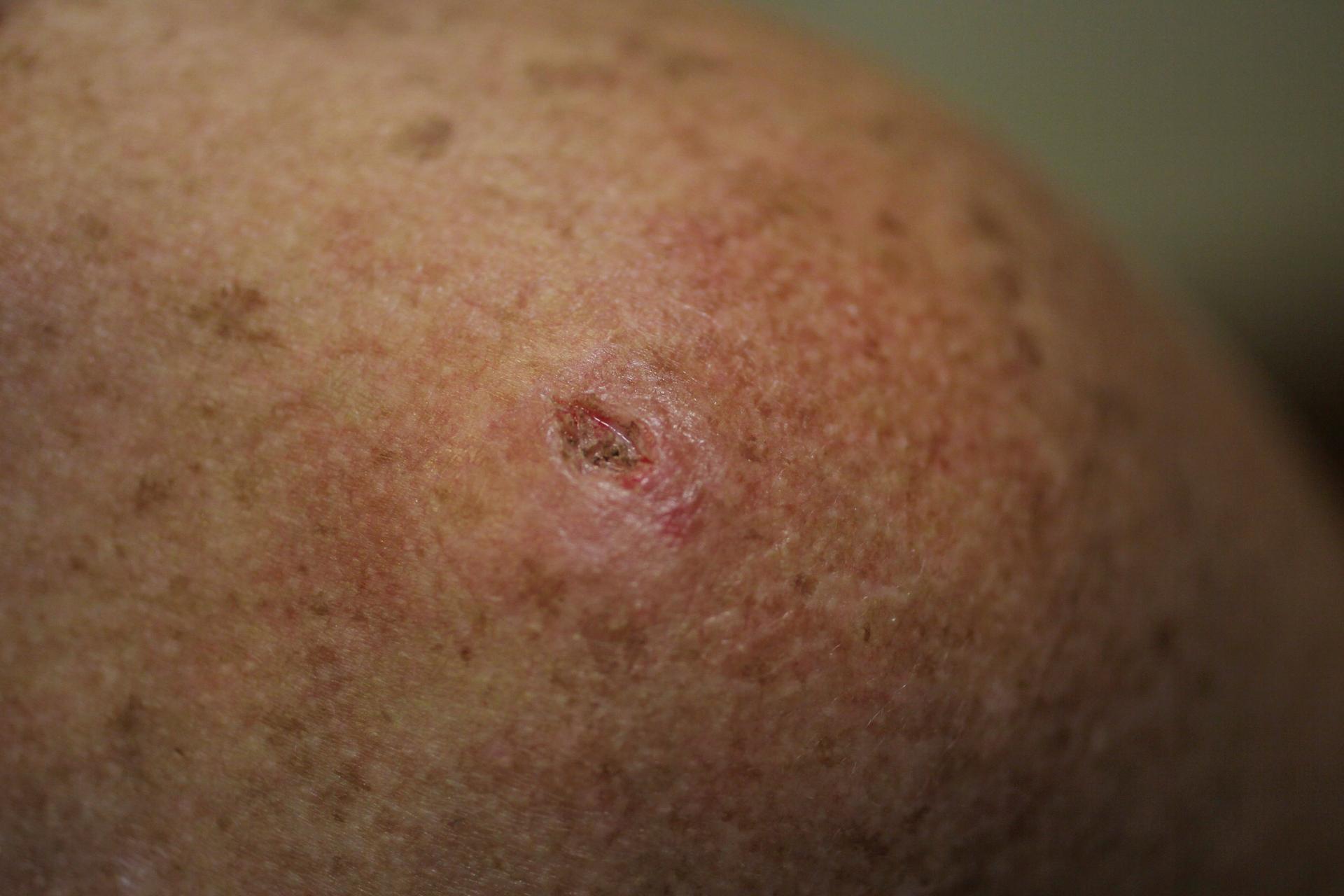
A patient has a mark on her skin after a biopsy was performed on a lesion to check for cancer due to sun exposure on June 15, 2011 in Miami, Florida.
The US Food and Drug Administration has approved a new drug, called Erivedge, for the most common type of skin cancer, basal cell carcinoma.
The once-a-day pill, to become available over two weeks, is made by Genentech, a unit of Swiss drugmaker Roche.
It is intended to treat locally advanced cancer for those patients who are not candidates for surgery or radiation, and also for patients whose cancer has spread locally or metastasized to other parts of the body, CBS reported.
It costs $7,500 per month and $75,000 for a typical 10-month course of therapy, according to the Boston Globe.
The FDA fast-tracked approval because there are no approved treatments for basal cell carcinoma, the AP reported, adding that:
The drug's label will warn that it is linked to fetal death and severe birth defects when it is used by pregnant women. The most common side effects of Erivedge include muscle spasms, hair loss, weight loss, diarrhea, fatigue, changes or loss in sense of taste, decreased appetite, constipation, and vomiting.
Lexington, Mass. company Curis Inc received a $10 million payment for collaborating with Genentech on the drug.