Bacterial ‘Hunger Games’ could help in the fight against infectious diseases
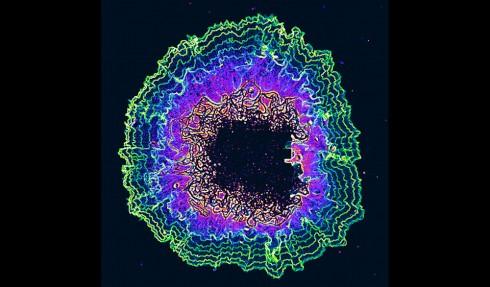
University of California, San Diego biologists imaged oscillations (shown as colored contour lines) from a growing biofilm (at center).
Infectious bacteria have a way of outsmarting us. So maybe it's time, scientists say, that we stopped trying to kill them and instead pit them against each other in a sort of bacterial Hunger Games.
“Bacteria, even though they are technically unicellular organisms, congregate and live in very tightly-packed communities, which we call biofilms,” explains Gürol Süel, an author on the study and an associate professor of molecular biology at the University of California, San Diego.
“They naturally form these collectives as a way of helping the colony survive,” he says. “And it seems that as a unit, as a community, they are much stronger in terms of surviving attacks — either chemical attacks or antibiotics — than they would be if all the bacteria would be on their own.”
Biofilms have been around for about 3.5 billion years and are found everywhere, Süel says — on our skin, within our digestive system (almost all the bacteria in the gut reside within such biofilms), in the depths of the ocean and on the tops of mountains.
When a biofilm forms, some cells find themselves on the interior of the community and some cells find themselves on the periphery. Since they are in different locations within this community, they experience different types of environmental conditions, Süel says, and they begin to communicate with each other by sharing or competing for metabolites.
The cells on the periphery form the first line of defense agains outside attack, protecting the cells on the interior, Süel says. But they also get first dibs on all the food that's coming in from outside the cell.
In return for the protection, the cells in the interior make a molecule called ammonium that the outer cells use for growth. As the outer cells absorb the ammonium, they continue to grow. But when they grow too much, they consume all the food, leaving none for the cells on the interior.
This is when things get interesting: when those outer cells begin to hoard the food, the interior cells stop making the ammonium gas molecule, which means the peripheral cells stop growing. When they stop growing, they stop consuming, which allow more food to slowly trickle inside the cell, allowing the cells on the interior to grow again. As a result, the interior cells resume making the ammonium, which allows the peripheral cells to grow again.
“Around and around you go,” Süel says. “You find these sort of beautiful oscillations.”
Süel and his co-authors figured out that if they were to artificially supply the ammonium metabolite to the outer layer of cells, they would grow independently of the interior cells and would no longer need them.
“Since the bacteria have no brains — they’re not doing this consciously, it's something that sort of emerges out of the complexity of the system — they would keep growing and by doing so, they would starve the interior cells that are the most protected and the most sheltered from attack,” Süel explains. “They would starve them to death. That's where the Hunger Games idea comes in.”
When they tested it out, it worked. Süel and his team genetically altered the exterior cells to allow them to synthesize the ammonium molecule on their own. As they had hoped, the outer cells kept growing and growing and then “kill[ed] the guys that are hard for us to reach,” Süel says. “They did the dirty job for us.”
They were “happily surprised,” Süel says. “Sometimes you know, you need luck in science, and we got a little bit lucky. It seems like it's really working.”
This article is based on an interview that aired on PRI's Science Friday with Ira Flatow